To answer this question it is first necessary to understand what Osmosis is.
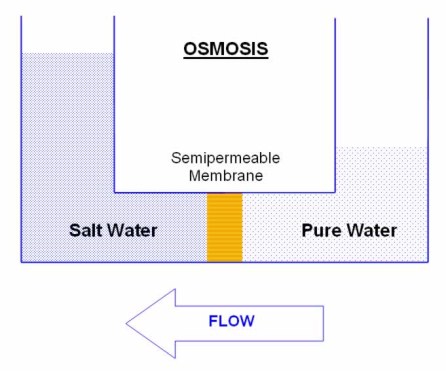
Osmosis
Osmosis is a natural process that occurs in all living cells. Water permeates through a membrane that excludes suspended solids, dissolved salts and larger organic molecules. These semipermeable membranes have pores of approximately 0.0005 microns in size.
Water molecules have a stronger tendency to escape from pure water than from a salt solution. Water flows through the semipermeable membrane from the pure solution to the salt solution in an effort to equalise the osmotic pressure of the two solutions.
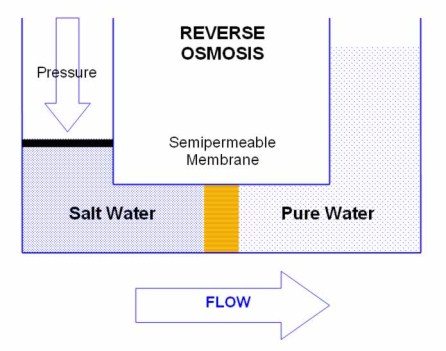
Reverse Osmosis
The Osmotic process may be reversed by applying pressure to the salt solution. In Reverse Osmosis, water from the salt solution is forced back through the semipermeable membrane to the pure solution. The process stops when the osmotic pressure of the increasingly salty solution equals the applied pressure.
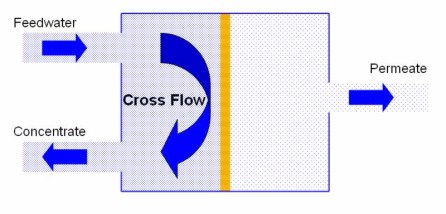
Membrane Design
In practice the salt solution must be continuously replaced before the osmotic pressure rises significantly. This is achieved using a cross flow mechanism where the surface of the semipermeable membrane is continually flushed. Therefore, commercial membranes have an inlet stream and two outlet streams. The inlet is known as the Feedwater and the outlets are the Permeate (pure water) and the Concentrate (reject water).